 Author: John Fletcher, Technical Support Manager
Related Product(s)>>
>>Download Full PDF
ABSTRACT
The determination of the concentration of soluble salts on a surface prior to the application of paint is a key inspection task for most protective coating processes. The entrapment of soluble salts, particularly on a steel surface can lead to premature coating adhesion failure and corrosion cells being established. This leads to a severe reduction in the expected life of the protective paint system.
SSPC Guide 15 describes the saturate special filter paper conductivity meter methodology amongst others for the retrieval and analysis of soluble salts from steel and other nonporous surfaces. There have been recent developments in the design and features of the equipment available for this specific test.
This test method has been used for many years and the basic method for extracting the soluble salts from the surface using a cleaned filter paper and pure water is unchanged. However, the electronic conductivity meter has been redesigned to include a menu structure with the measurement sequence displayed and a reading memory to store the values obtained using this test. The data contained in the memory can be transferred to Data Management Software to automate the presentation of the results in reports.
This paper describes the measurement method and presents a case study of the analysis of test panels to demonstrate the performance of the method.
INTRODUCTION
The current version of SSPC Guide 15, Field Methods for Retrieval and Analysis of Soluble Salts on Steel and Other Nonporous Substrates was first published in June 2005. This Guide describes the most commonly used field methods for the retrieval and analysis of soluble salts on steel and other nonporous substrates. SSPC Guide 15 was revised in 2013 and the current version was published in August 2013.
The Guide states that coatings applied on surfaces contaminated with soluble salts exceeding a certain concentration exhibit diminished performance and that the Guide is intended to assist the user in selecting specific procedures for retrieving and analyzing soluble salts.
Within the Guide, Clause 4.2.3: The Filter Paper Extraction Method is described as
follows:
This method incorporates soluble salt extraction with water-saturated filter paper and analysis using conductivity. The paper wets the surface and extracts soluble salts through absorbance. After a pre-determined time, the paper is removed from the surface and placed over the electrodes of a proprietary concentric ring conductivity meter. The meter indicates the conductivity of the wetted paper.
A new design of conductivity meter based on microprocessor electronic circuitry has been developed with features to make the assessment of surface contamination by soluble salts quick and easy.
THE SATURATED FILTER PAPER EXTRACTION METHOD
Soluble salt measurements in general require two processes, the first is to extract a test solution with the salts from the surface, the second is to analyse the solution to determine the concentration of the salt on the surface.
In the case of the saturated filter paper extraction method, cleaned filter papers are wetted with a controlled volume of pure water and the paper is then placed on the surface to extract the soluble salts. The paper is left on the surface for 2 minutes and then it is removed from the surface and placed on the electrode of the conductivity meter. The meter then tests the conductivity for this known area of the filter paper and the known volume of test solution. The result is displayed by the gauge as a value in μg/cm². Figure 1 shows a schematic view of the measurement process for the saturated filter paper extraction method.
The soluble salt concentration includes the weight of both cation and anion. The reading is therefore derived from a total conductivity measurement. The gauge converts the total conductivity reading over the known area of the sample paper, calibrated as sodium chloride. To convert the reading to a chloride ion value assuming that sodium chloride is the only source of chloride ions, the factor will be 1.65 based on atomic weights. Therefore a reading of 2.0 μg/cm² total salt level on the instrument would be divided by 1.65 to give 1.2 μg/cm² as chloride.
|
Figure 1 - The saturated filter paper extraction method
|
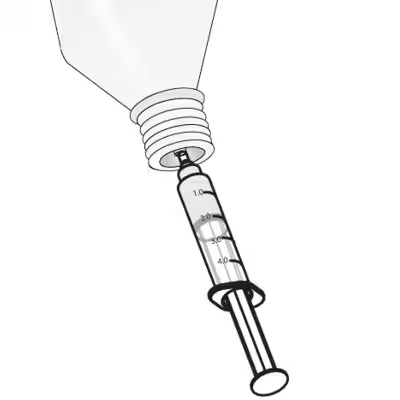 |
Fill the syringe with 1.6 ml of high purity water.
Note: Non-pure water (up to 2µg/cm²) can be automatically offset with the gauge.
|
|
|
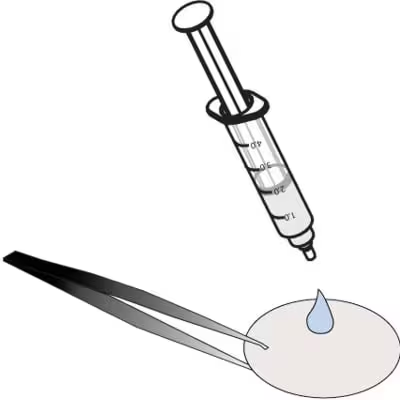 |
Eject the 1.6ml on to a clean unused sample paper, taking care to retain all the water on the paper.
|
|
|
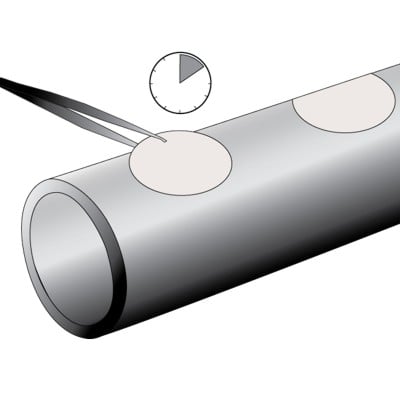 |
Place wetted paper on to the area under test, pressing firmly into contours and irregularities to remove any entrapped air.
Start the timer on the gauge. Whilst waiting for the sample time to elapse, additional tests can be prepared.
|
|
|
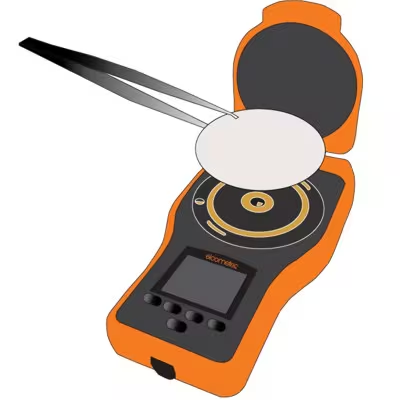 |
After 2 minutes, remove the paper from the surface and place it on to the gold-plated electrodes.
|
|
|
 |
Close the lid, ensuring that the magnetic catch is fully engaged.
The reading will automatically be displayed and stored into memory together with paper size, temperature, date and time.
|
|
|
 |
The reading will be displayed.
|

Figure 2 – The Main Parts of the Salt Contamination Test Kit
DESIGN FEATURES OF THE NEW FILTER PAPER CONDUCTIVITY METER
In line with several modern electronic coating inspection gauges, the latest design of conductivity meter makes use of microprocessor electronics to enable operational features to be added to make the measurement of surface salt concentration and the management of the resulting data quicker and easier. Microprocessor electronics allow the gauge to be operated from a menu displayed on a large color LCD, with on screen charts and step-by-step guidance for the user in different languages and four large buttons that change their function depending on the area of the menu selected.
The gauge case is hand-held and fully portable for field use and is designed to be dust and water resistant to IP64 equivalence. The gauge uses large control buttons so that the gauge is easy-to-use when wearing gloves.
The benefits of the filter paper conductivity meter include:
- Measuring range up to 50 μg/cm² (3000ppm)
- Measurements can be displayed in six different units: μg/cm², ppm, μS/cm,
S/cm, % Salinity and mg/m²
- Fast reading rate allows multiple tests to be completed efficiently
- Pressure plate ensures a constant and uniform pressure to paper
- Calibration verification tiles for field use, three values (optional)
- Automatic detection of paper size and adjustment of reading value
- Automatic temperature compensation ensures accurate results
- Off-set calibration adjustment for use with less than pure water
- Enables date and time stamp, temperature and size of test paper to be recorded
- USB and Bluetooth® data output to data management software
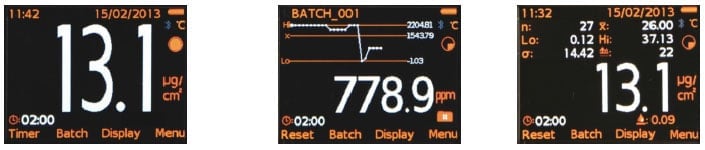
Figure 3 Examples of Displays Showing (L) Reading, (C) Run Chart for last 20 readings in Parts Per Million and (R) Statistics from a Batch of Readings
FIELD VERIFICATION
Field verification of all inspection gauges is an important issue. For example, the ASTM D7091-2012, Standard Practice for Non-destructive Measurement of Dry Film
Thickness of Non-magnetic Coatings Applied to Ferrous Metals and Non-magnetic, Non-conductive Coatings Applied to Non-Ferrous Metals, now includes a verification
of the coating thickness step prior to gauge use. This recognize that calibration of the gauge is carried out by the manufacturer or an approved service centre; gauges
are adjusted for the conditions of use and then verified before use.
The same is true for the soluble salt meter. Historically, salt contamination gauges, e.g. the SCM 400, have been calibrated by testing them with a series of solutions
with known quantities of sodium chloride dissolved in them. A series of tests were carried out noting the readings for each concentration of salt. Figure 4 shows the
levels of contamination tested and a typical reading for the gauge for a calibration certificate.
| Figure 4 – Table of Calibration Values for Original Design of Filter Paper Conductivity Meter |
|---|
| Contamination Reference Values (μg/cm²) | Salt Contamination Meter Reading (μg/cm²) | Difference Between Reference and Reading (μg/cm²) | Gauge Tolerance |
|---|
| 0.0 | Salt Level Too Low | Not Applicable | Not Applicable |
| 0.2 | 0.2 | 0 | 0.18-0.22 |
| 0.5 | 0.5 | 0 | 0.45-0.55 |
| 1.0 | 1.20 | 0.2 | 0.9-1.1 |
| 5.0 | 5.20 | 0.2 | 4.5-5.5 |
| 10.0 | 9.60 | 0.4 | 9.0-10.1 |
| 15.0 | 13.60 | 1.4 | 13.1-14.1 |
| 20.0 | >20.0 | Not Applicable | Not Applicable |
Although the operating instructions for the older design of gauge have a method for
preparing a test solution for verifying the performance of the gauge, this is really a
laboratory procedure and does no lend itself to field verification. For example the test
solution needs to be freshly made and should not be stored prior to use.
The procedure for checking the SCM 400 gauge is as follows:
- Make up a solution of 31.6 mg (±0.1) of laboratory grade sodium chloride, dissolved in 100 ml of high purity water.
- Using precisely 1.5 ml of the solution in a syringe wet a filter paper and place it on the electrode.
- The gauge reading should be in the range 5 μg.cm-2 (±10%) at 25°C.
- For temperatures below 25°C apply a correction factor of -1.7%/°C.
For temperatures above 25°C apply a correction factor of +1.7%/°C.
The latest design of the gauge has verification discs that can be used in the field to check the performance of the gauge, as and when required. These discs each have a set of precision resistors selected and tested to give a specific conductivity value.

Figure 5 - Calibration Verification Tiles in use
The nominal values for the tiles are 0.4, 4.9 and 19.5 μg/cm², thus the operational range of the gauge is covered. The gauge prompts the user to place the tile on the measurement electrode as shown in figure 5. The gauge tile reading must then be compared to the value printed on the tile label and assessed against the tolerance allowed for the tile value, which is ± 0.15% of the reading. This tolerance is printed on the calibration certificate for the tile set.
For example, for a tile value of 19.5 μg/cm², the gauge reading must be between 19.2 and 19.8 μg/cm² for the gauge to be within tolerance. As is generally the case, when the gauge/tile readings are outside the stated accuracy for the gauge, re-calibration is recommended.
TEMPERATURE COMPENSATION
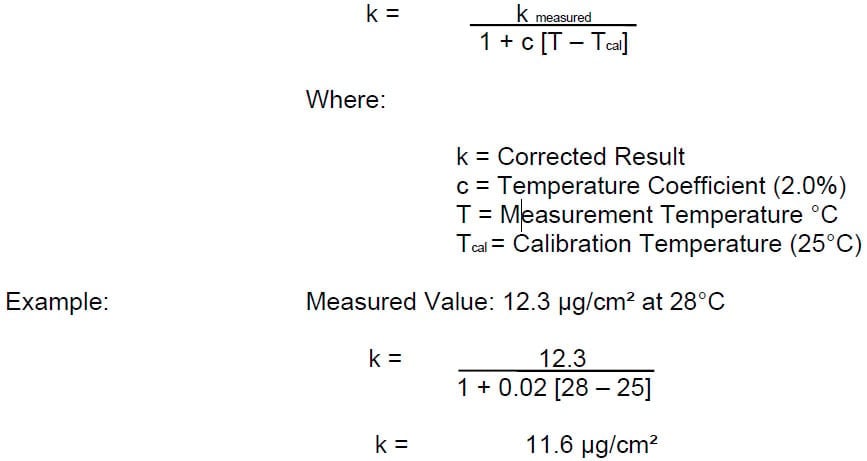
The temperature of the substrate and the water solution will have an effect on the reading of the conductivity. Gauges are calibrated at 25° C (77° F). In order to provide an accurate measurement of soluble salt contamination of the pure water the reading must be adjusted for the actual temperature of the test.
The new design of salt contamination gauge has a Temperature Compensation function which can be activated by the user and measures the temperature of the wet filter paper when it is placed on the measurement electrode and uses this value to adjust the reading.
The following equation can be used for gauges that do not have the Temperature Compensation feature.
DATA MANAGEMENT
The latest salt contamination gauge has the capability to transfer data from the gauge’s memory via a USB cable or wirelessly via Bluetooth® technology to personal computers or mobile devices which are Bluetooth® enabled and are running suitable data management software.
The data management program that is designed to operate with the new salt contamination gauge has a data transfer wizard to help the user make the connection to the gauge.
The memory of the gauge can be divided in to approximately 2,500 separate batches of readings, providing a total of up to 150,000 readings. Each batch can be allocated to a different area of the structure so that a complete picture of the structure can be created. These batches can each be given an alpha-numeric name for easy recognition and the data management software can organize these batches in to job
file folders for easy access.
The gauge is capable of statistical calculations on the data including:
- The number of readings taken in the batch
- The mean (average) of the batch
The standard deviation of the readings in the batch
- The lowest reading taken and the highest reading taken
- The Coefficient of Variation (COV); The COV is defined as the ratio of the standard deviation to the mean and is a normalized measure of dispersion of the batch data. When comparing batches with widely different mean values, the Coefficient of Variation should be used for comparison rather than the standard deviation.
- The number of readings above the high limit set, when a limit is set and activated.
The data management software has a simple Windows based file structure and the look and feel of an Outlook style program. There is a conventional wizard-based data import routine.
The software can be set up with project folders and data tags, photographs, GPS data and date and time options can be added to the data that is transferred from the memory of the gauges. The data can even be transferred directly in to a spread sheet such as Excel if required.
Once transferred, the data from the gauge can be managed utilizing the functions of the data management software. The batch data can be dragged in to a project folder. The individual readings can be viewed along with the statistics. Any limits imported from the gauge can be viewed and edited if required. Histograms, run charts and pie charts are calculated and can be displayed using the folder tags and notes can be added for clarity. Figure 6 shows one of the data management screens with an individual batch of salt contamination conductivity data, illustrated with a histogram to show the distribution of the readings within a single batch.
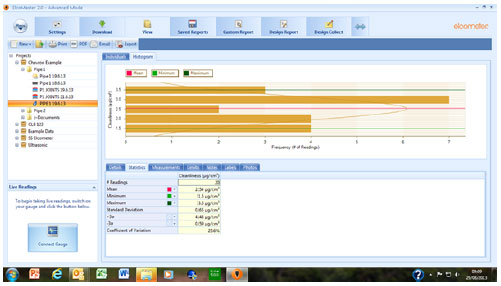
Figure 6 – Screen from Data Management Software Showing Salt Conductivity Data
A simple to use report designer tool allows measurements to be added to images of the component by location. Measurements and statistical information can be placed in scanned document formats and data can be combined either as batches from the same gauge or by combining measurements from different gauge type in to a single report. For example, profile height and salt contamination measurements after blasting can be combined with climatic data collected during the coating application and dry film thickness values after the coating has cured to form a comprehensive coating process report. The report can then be customize by adding company logos and other related information such as inspection procedures and specifications associated with the project.
ADVANTAGES AND DISADVANTAGES FOR THE FILTER PAPER EXTRACTION METHOD
Guide 15 identifies the main advantage of the Filter Paper Extraction Method as being that the filter paper procedure is relatively simple and is less subject to operator error than some of the other methods that are included in Guide 15.
The guide also points out that the limitations of the method are:
- The instrument measures total soluble salts, rather than a specific ion such as
chloride or nitrate.
- There is no independent data on the accuracy or precision of this method.
- The water is subject to evaporation loss under conditions of high temperature
and/or low humidity.
It should be noted that while different ratios of soluble salts (chlorides, sulfates or nitrates) may be present on a surface depending on the location (marine, urban or rural environments), these ionic salts have a detrimental effect on the performance of protective coatings.
Most specifications require that the level of salt on the surface to be painted to be < 5.0 μg/cm²nd some specifications, NORSOK M-501 now require < 2.0 μg/cm² for marine service.
It should be noted that 2.0 μg/cm² is equivalent to 20 mg/m². Some specifications use the mg/m² units for surface soluble salt contamination values.
It has proved difficult to create a known reference surface on a blasted steel substrate with which to compare the different salt contamination measurement systems. The assessment of the analysis methods can be carried out using known solutions of the appropriate salts in pure water but these solutions cannot be used for the determination of the extraction efficiency of the methods when removing salts from the steel surface.
An evaluation of the Saturate Filter Paper Extraction method carried out by an independent team in Saudi Arabia reported the following benefits:
- The method is suitable for a wide range of shapes, orientations, surfaces and
finishes.
- It is quick and simple to use.
- Battery operated and portable.
- Confirms adequate cleaning of surfaces before coating, aiding the prevention of premature coating failure.
- Reveals the build-up of salts on venerable surfaces, which can be cleaned to increase the lifetime of a coating.
- Test papers can be re-moistened to obtain a similar test result to that originally obtained for confirmation of the test as proof of inspection and its purpose.
- Fast reading rates allow multiple tests to be completed.
- The pressure plate ensures a constant and uniform pressure to be applied to the paper.
- The gauge will automatically detect the paper size and automatically adjusts the conductivity value to compensate.
- Non-oxidising gold-plated contacts ensure lifetime accuracy of the readings.
CONCLUSIONS
Digital data management has become an important aspect of coating process inspection as it saves time and improves accuracy, allowing important information to be made available to all interested parties quickly.
The most recent development of the conductivity gauge for the saturated filter paper method for soluble salt determination on surfaces prior to the application of coatings has brought this method in to the group of gauges with data management capabilities.
The saturated filter paper method for soluble salt determination has been used for several years and is one of the methods referenced in the SSPC Guide 15. The main advantage of this method is the simplicity and ease of use.
An independent evaluation of the method, using the new design, has confirmed the benefits of this method outlined in SSPC Guide 15.
As with several other salt contamination determination methods, a reference standard for a soluble salt contaminated steel surface is required to fully assess the accuracy of the method and work is continuing to develop a method to produce such a reference standard.
Related Product(s)Download Full PDF |